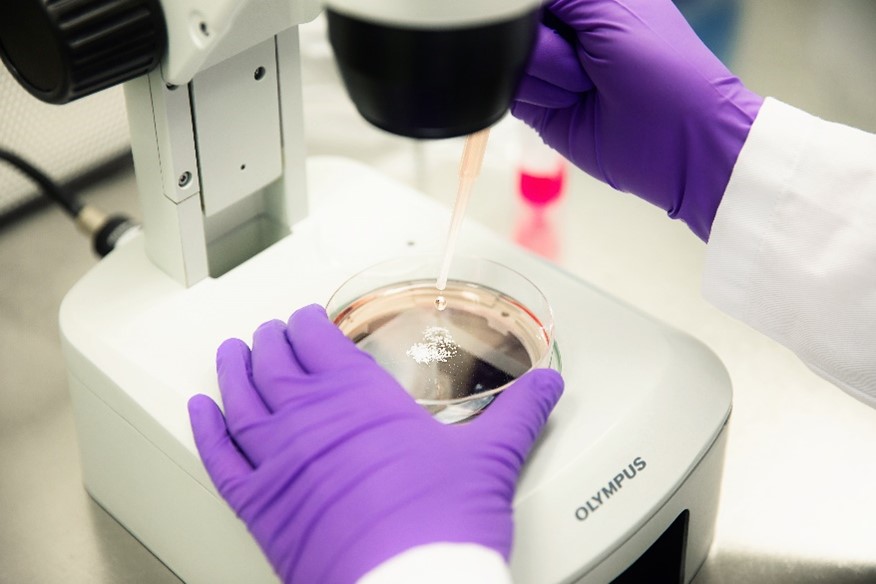
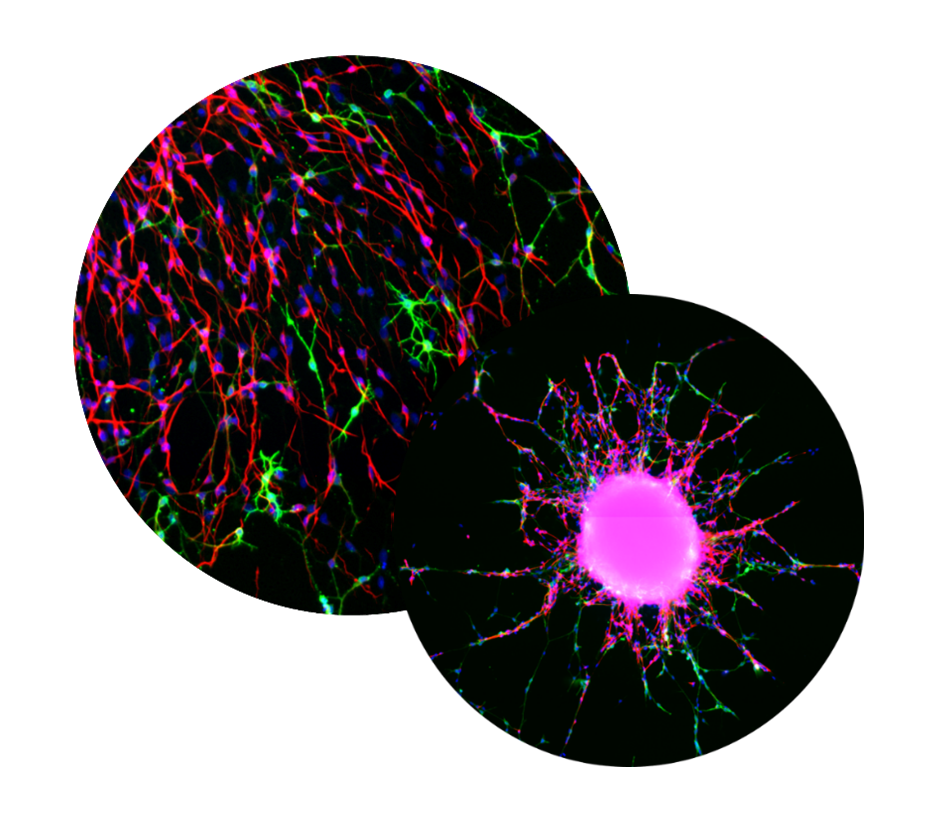
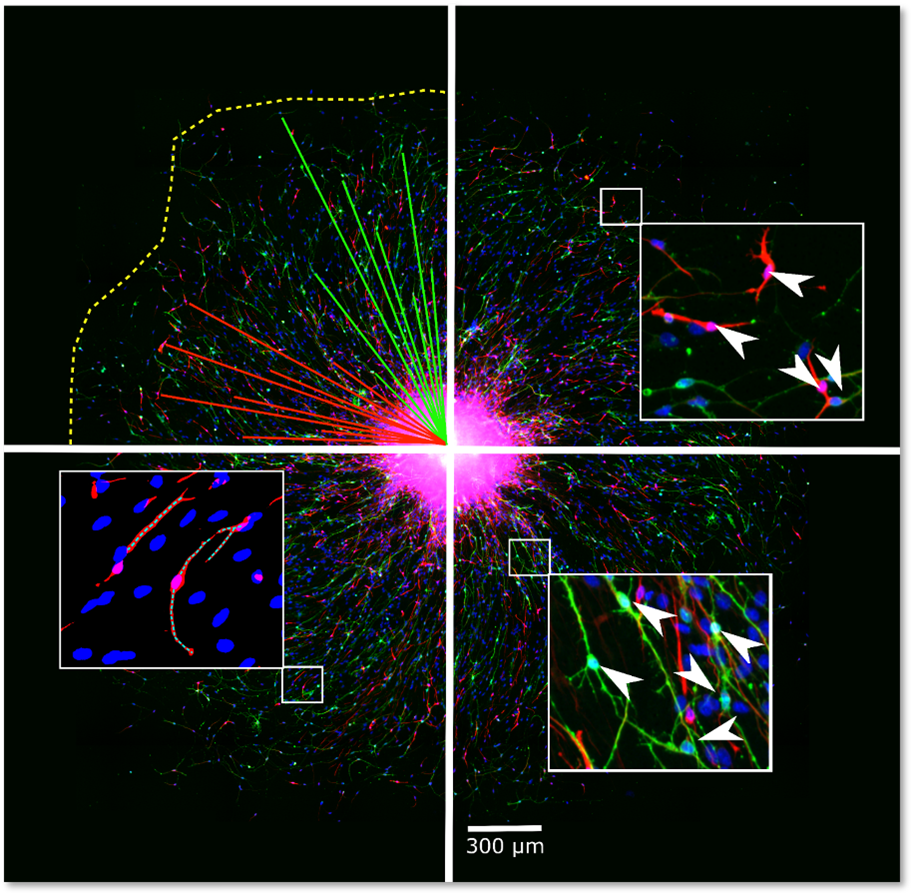
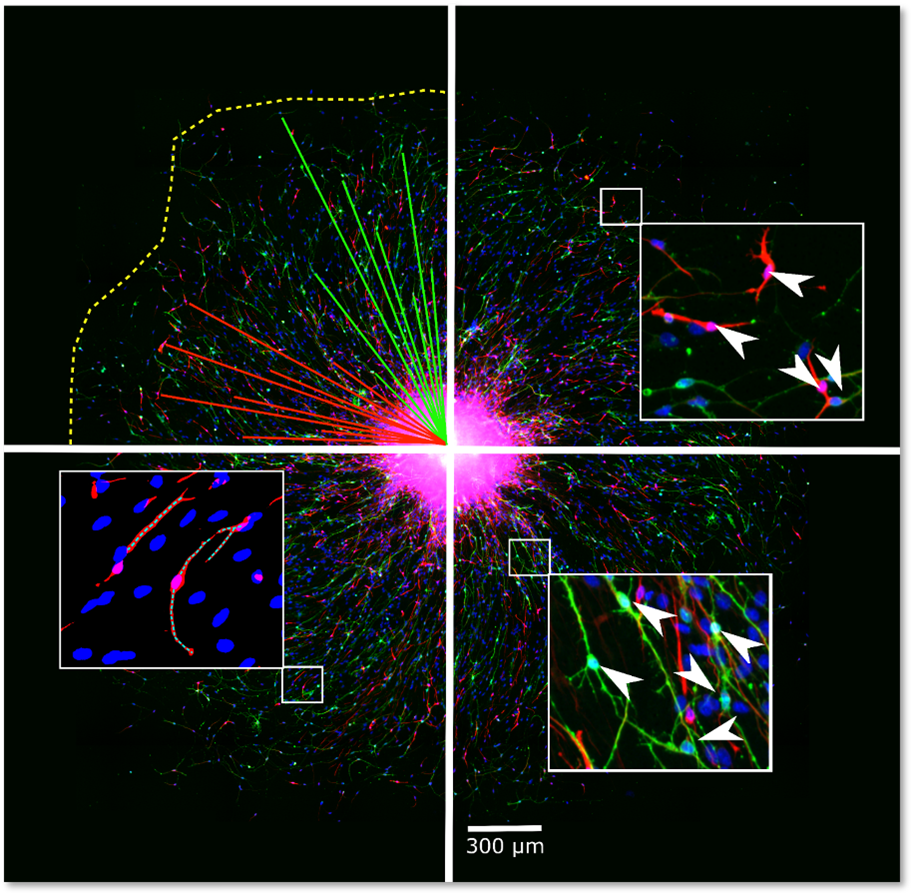
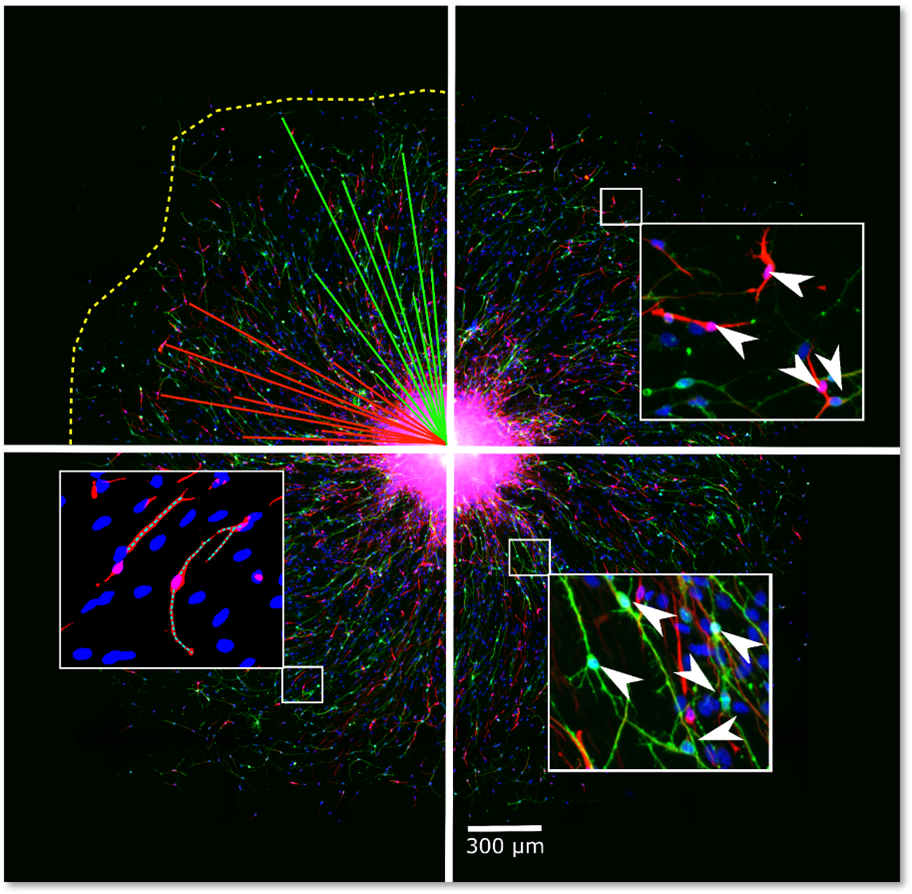
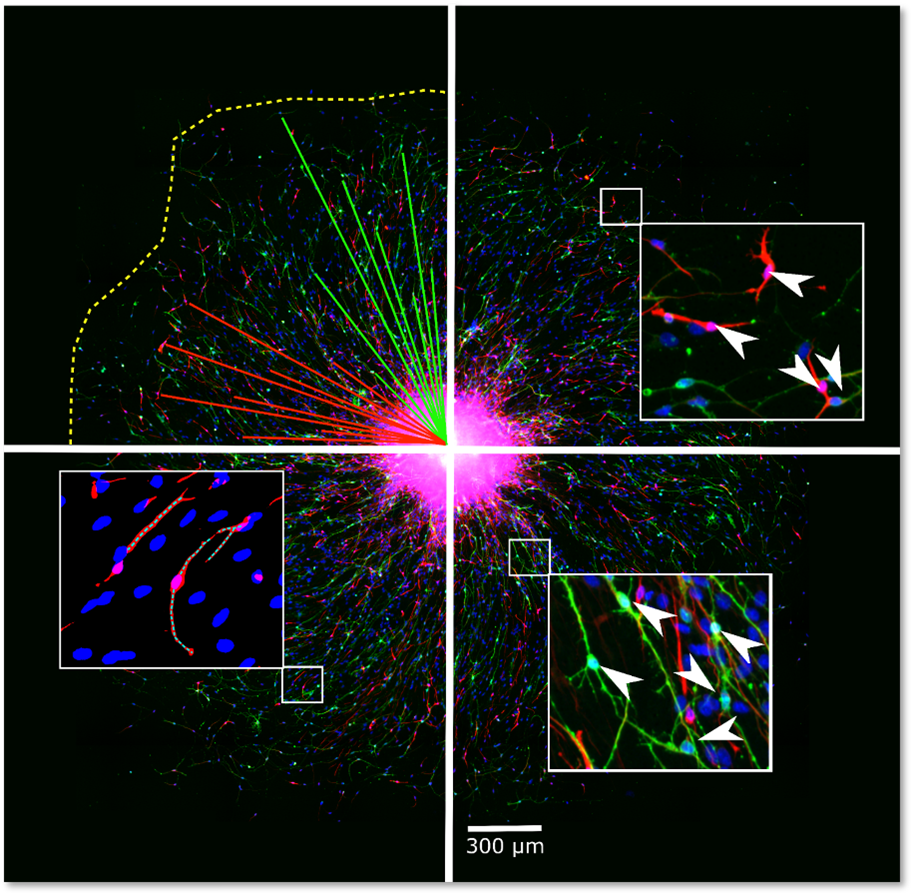
NPC proliferation is a fundamental neurodevelopmental process that, when disturbed, like in Zika virus-infected primary NPC, leads to microcephaly in children. The proliferation of primary hNPCs of fetal origin or human induced pluripotent stem cells (hiPSCs) grown as neurospheres in 3D is studied by measuring an increase in sphere size over time and/or by measuring DNA synthesis as BrdU incorporation in vitro.